OBJECTIVE
A depyrogenation tunnel is a crucial piece of equipment in the pharmaceutical industry, particularly in the production of injectable drugs. Its primary objective is to remove or reduce pyrogen contamination from glassware, containers, and other equipment used in the production of sterile pharmaceutical products. Pyrogens are fever-causing substances that can cause adverse reactions in patients if they enter the bloodstream during drug administration. By eliminating these contaminants, the depyrogenation tunnel ensures the safety and efficacy of injectable pharmaceutical products.
1. Introduction: What is a Depyrogenation Tunnel?
A depyrogenation tunnel is a continuous dry-heat sterilization system designed to remove pyrogens (endotoxins) and achieve sterility for containers like vials and ampoules prior to aseptic filling. These tunnels are critical in sterile dosage form manufacturing, ensuring that no microbial toxins enter parenteral drugs.
💡 Pyrogens, especially bacterial endotoxins, can cause severe febrile reactions in patients if not removed from injectables.
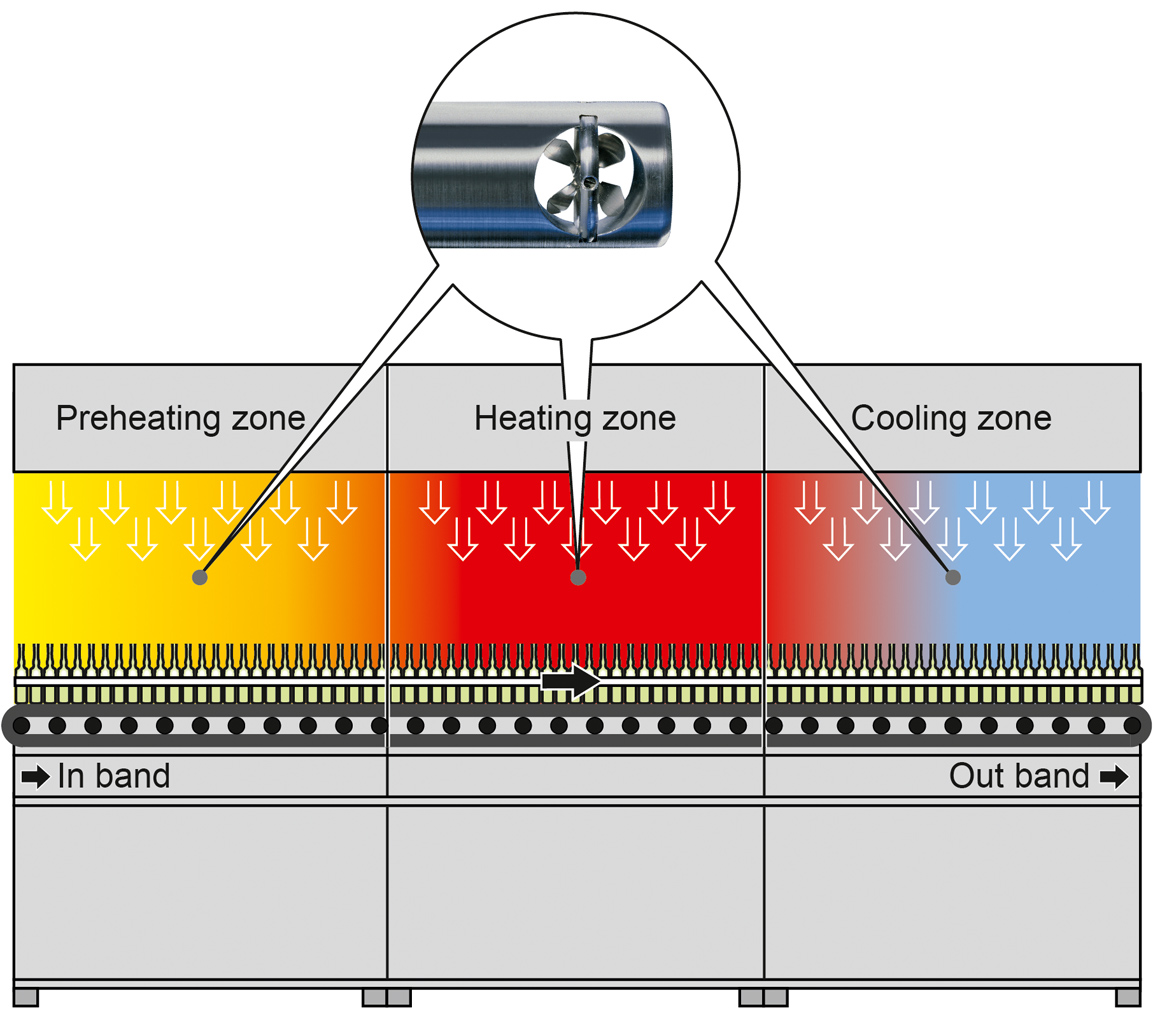
[Insert Diagram: Exterior view of depyrogenation tunnel with infeed, sterilizing, and cooling zones]
2. Working Principle: Dry Heat Depyrogenation
Depyrogenation tunnels use high-temperature dry heat (typically ≥250°C) to inactivate or destroy endotoxins. Unlike steam sterilization, which denatures proteins using moisture, dry heat decomposes lipopolysaccharides (LPS) through oxidation.
Here’s a step-by-step overview of the process:
-
Loading: Equipment such as glass vials and containers are loaded onto a conveyor belt.
-
Pre-heating Zone: The equipment passes through a pre-heating zone to ensure uniform temperature before entering the main heating zone.
-
Heating Zone: The equipment is exposed to high temperatures, typically ranging between 250°C to 400°C, for a specific period, usually between 15 to 45 minutes. This heat treatment destroys bacteria and other microorganisms and removes pyrogens.
-
Cooling Zone: After the heating process, the equipment passes through a cooling zone to lower its temperature to a safe level before being unloaded from the conveyor belt.
This process ensures that the equipment used in pharmaceutical manufacturing is free from pyrogens, minimizing the risk of contamination and ensuring the safety and efficacy of the final pharmaceutical product.
3. Design & Construction Features
Depyrogenation tunnels consist of three primary zones:
|
Zone |
Function |
Approx. Temp |
| Infeed/Pre-Heat | Evaporates surface moisture | ~100–160°C |
| Depyrogenation | Destroys endotoxins (e.g., 3-log) | ≥250°C |
| Cooling | Protects vials before filling | <60°C |
Other Design Elements:
– Stainless steel AISI 316L surfaces
– HEPA filters (≥ H14) for LAF
– Air curtains to prevent cross-contamination
– Automatic conveyor with speed control
4. Qualification & Validation
Depyrogenation tunnels undergo 3 major validation stages:
Installation Qualification (IQ): Verifies proper installation, component specs, calibration of sensors, wiring.
Operational Qualification (OQ): Confirms functional performance: airflow velocity (90FPM +/- 20%), temperature uniformity, alarms, belt speed.
Performance Qualification (PQ): Endotoxin challenge test using vials spiked with known endotoxin to confirm 3-log or 12-log reduction.
5. Acceptance Criteria
|
Parameter |
Criteria |
| Depyrogenation | ≥3-log (routine) or ≥12-log (validation) |
| Min. tunnel temperature | ≥250°C |
| Holding time | 30 minutes at peak temp (typical) |
| HEPA efficiency | ≥99.997% @ 0.3 μm |
| Belt speed | Validated to ensure dwell time |
| Airflow pattern | Laminar, unidirectional |
6. Critical Process Parameters
To ensure validated performance, the following must be tightly controlled:
– Tunnel temperature profile: Must be uniform across all vials.
– Conveyor belt speed: Directly affects dwell time.
– Zone segregation: Prevents recontamination.
– HEPA filter performance: Ensures ISO 5 environment.
– Alarm systems: Must flag deviations in temperature, airflow, or speed.
7. Regulatory Guidelines & Standards
|
Guideline |
Key Focus |
| USFDA (21 CFR Part 211.94) | Equipment must prevent contamination |
| EU GMP Annex 1 (2022) | ISO 5 environment, validated sterilization |
| WHO GMP | Dry heat standards for depyrogenation |
| ISO 14644-1 & 2 | Cleanroom classification & monitoring |
| PIC/S PI 008 | Qualification and validation principles |
8. Routine Monitoring & Maintenance
Daily:
– Monitor temperature sensors, conveyor speed
– Inspect HEPA filter pressure drops
Once in Three Months:
– Preventive Maintenance (PM) as per OEM
Once in Six month:
– HEPA filter integrity test (e.g., DOP/PAO)
– Calibration of temperature probes
– Requalification (PQ)
Conclusion
A depyrogenation tunnel is a critical piece of equipment in sterile manufacturing. It must be carefully designed, qualified, and continuously monitored to meet global GMP standards and ensure patient safety.
Useful links
