Lyophilization, also known as freeze-drying, is a method used in pharmaceutical and food industries to preserve sensitive materials. Here’s a detailed overview:
Lyophilization Process
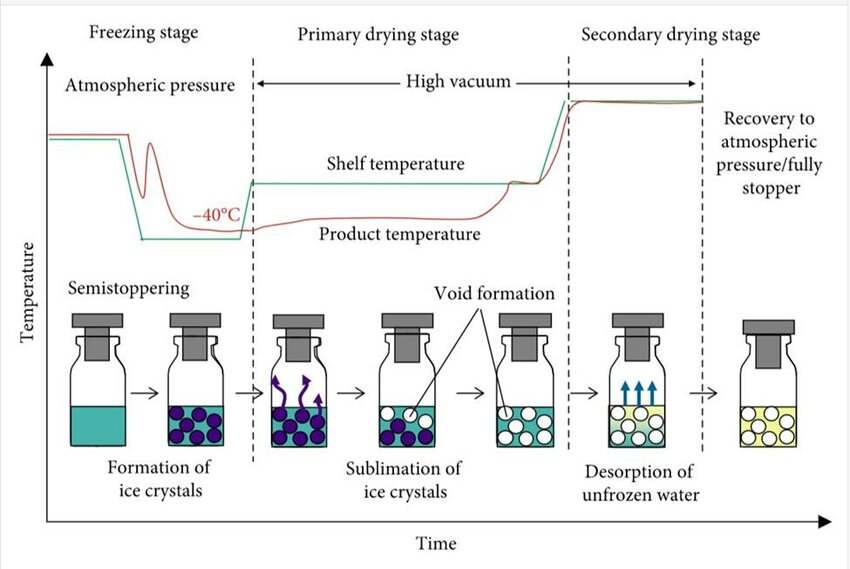
The process involves three main steps:
- Freezing: The product is frozen below its eutectic point, ensuring the solvent (usually water) solidifies completely. This step stabilizes the material and prepares it for drying.
- Primary Drying (Sublimation): Under low pressure (vacuum), the frozen solvent sublimates directly from solid to vapor without passing through the liquid phase. This helps retain the product’s structure.
- Secondary Drying (Desorption): The remaining bound water molecules are removed under higher temperatures, achieving the desired moisture content.
Objective
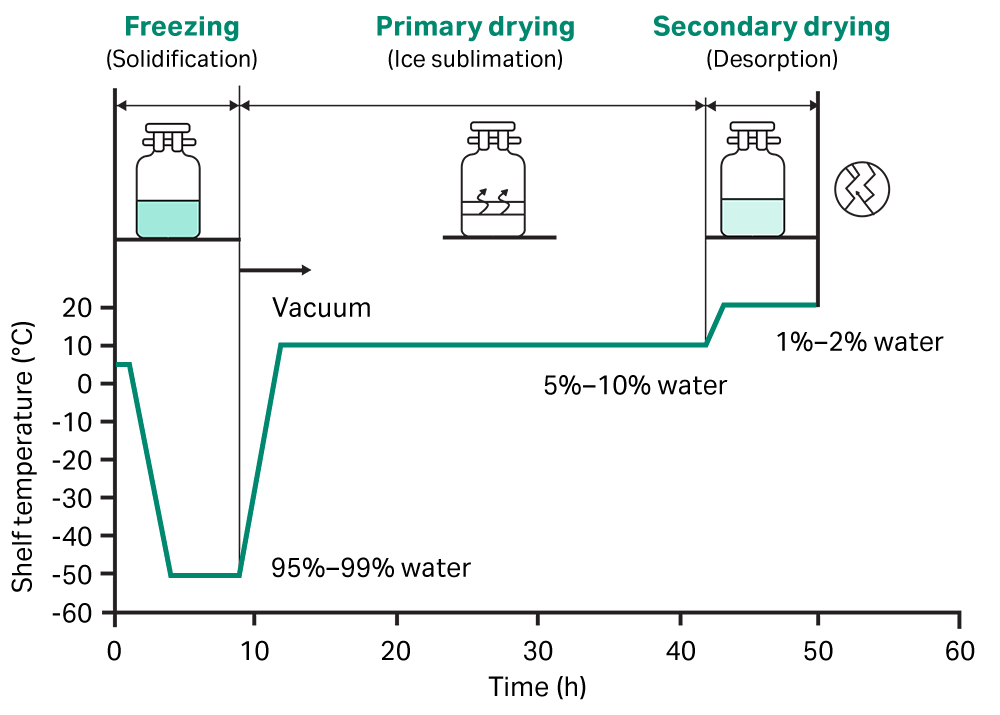
- Preservation: Maintain the integrity and efficacy of sensitive products, such as pharmaceuticals (e.g., injectables like vials or ampules) and food items.
- Extended Shelf-Life: Minimize microbial growth and chemical degradation over time.
- Convenient Storage and Transport: Ensure stability without refrigeration for easy distribution.
Benefits
- Retention of Product Quality: Lyophilized products retain their original structure, potency, and composition.
- Long Shelf-Life: Prevents degradation and spoilage, enhancing longevity.
- Ease of Reconstitution: Rapidly restored by adding water or solvent when needed.
- Lightweight: Reduced weight and volume make it convenient for transport.
Demerits
- Cost Intensive: Requires specialized equipment and significant energy for freezing and vacuum creation.
- Time-Consuming: The multi-step process can be slow and resource-intensive.
- Complexity: Requires skilled personnel for monitoring and optimizing each phase.
- Potential Product Damage: Improper freezing or drying parameters might lead to structural damage or loss of activity.
It’s widely used in pharmaceutical manufacturing, particularly for injectable drugs in regulated markets like USFDA and MHRA, making it a crucial process in facilities like the one you oversee.
How it works the Lto
Credits: Tofflon Group
Lyophilization process nicely explained by GEA Group
2 responses to “LYOPHILIZATION”
-
“Congratulations on launching this incredible pharma technical information platform! Your dedication to providing accurate, up-to-date, and insightful industry knowledge is truly commendable. This website will undoubtedly serve as a valuable resource for professionals and enthusiasts alike. Wishing you great success in this endeavor!”
-
Thanks for your comments and support
-

“Congratulations on launching this incredible pharma technical information platform! Your dedication to providing accurate, up-to-date, and insightful industry knowledge is truly commendable. This website will undoubtedly serve as a valuable resource for professionals and enthusiasts alike. Wishing you great success in this endeavor!”
Thanks for your comments and support